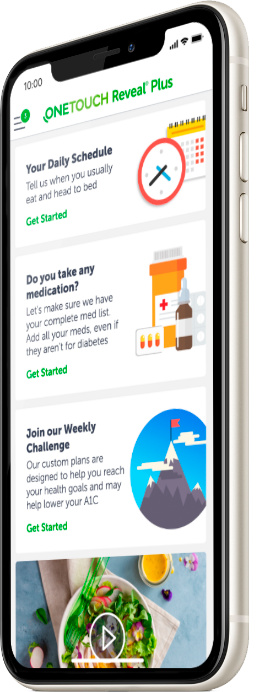
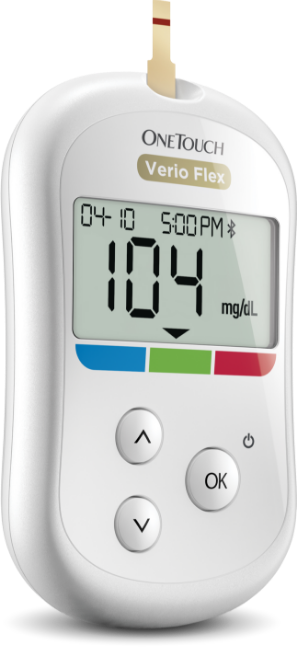
Reduced workplace productivity and absenteeism accounts for roughly $30 billion per year of diabetes costs. Better daily self-care, improved lifestyle changes, and enhanced doctor-patient communication are keys to helping your employees live well with diabetes.
A Digital Coach for Daily Care
Empower your employees with features designed to build and improve their daily diabetes management skills:
- Glucose monitoring and medication alerts
- Healthy eating and exercise habits
- Immediate guidance to minimize related risks
Continuous Support
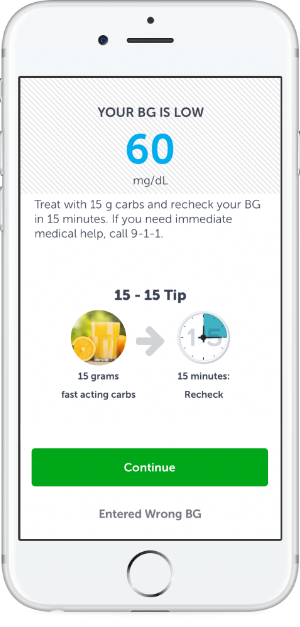
AI technology delivers customized digital coaching to instantly guide employees on how to address hypo and hyperglycemic readings.
Healthier Lifestyles
Actionable dietary, activity, and blood pressure tips help to address hypertension and obesity.
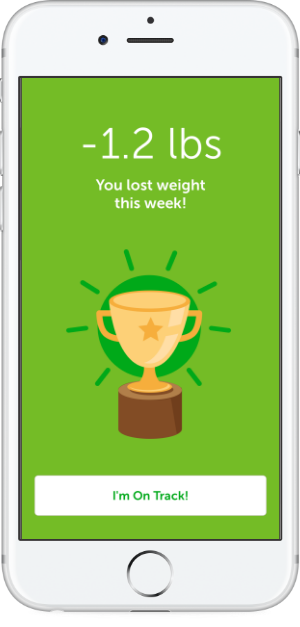
Higher Productivity
Better daily diabetes management could mean less unplanned absences, keeping your costs and their day on track.
A Digital Coach for Daily Care
Empower your employees with features designed to build and improve their daily diabetes management skills:
- Glucose monitoring and medication alerts
- Healthy eating and exercise habits
- Immediate guidance to minimize related risks
Testing the Relationship Between A1C Reduction and Cost Savings in Type 2 Diabetes
Welldoc provided starting and ending A1C data from 3,000 BlueStar® users to IBM Watson for a retrospective analysis funded by Welldoc. IBM Watson mapped this data onto their patient claims database to estimate cost savings associated with utilizing BlueStar®. Documented A1C reductions associated with BlueStar® are correlated with cost differences associated with the following factors.
Hospitalizations & ER visits
Cost of supplies & pharmacies
Cost of comorbid complications
Where did the data come from?
Claims data from patients in the IBM Watson’s MarketScan Database, which captures data of more than 80 million individuals.
What did the study find?
Once the patients were stratified by A1C band, the analysis was run through the commercial and the medicare sector. For an overview on A1C and what it means click here.
Learn More
Add the OneTouch Reveal® Plus app to your diabetes care portfolio today.
Studies in Type 2 diabetes
3-month randomized-controlled clinical trial
In a prospective, randomized, controlled study, Quinn et al. (Diabetes Technology and Therapeutics, 2008) found that individuals whose A1C levels were greater than or equal to 7.5% within most recent three months and received Welldoc’s software experienced an average decrease in A1C of 2.03%. Patients in the control group obtained an average A1C reduction of 0.68% (P < 0.02, one-tailed). Of the intervention patients, 84% had medications titrated or changed by their HCP compared to controls (23%, P = 0.002).
12-month randomized-controlled clinical trial
In a prospective, randomized, controlled study, Quinn et al. (Diabetes Care, 2011) found that, individuals with type 2 diabetes whose A1C levels were poorly controlled (>9.0%) or abnormal (7.5-8.9%) at the time of enrollment, and who were randomized to use a mobile phone app to help them manage their diabetes in addition to usual care, improved A1C by an average 1.9%, compared with 0.7% improvement in persons randomized to usual care alone, a difference of 1.2% (P < 0.001) over 12 months.
Data-Driven Technology Meets the Rising Cost of Diabetes
Improve Employee
Health
More than 40 peer-reviewed posters and publications highlight the value of the OneTouch Reveal® Plus app. Learn more about these publications on Welldoc.
Cost-Effective
Care
Estimated annual cost savings ranging from $1,824 - $5,244 per user resulting from managing glycemic variability2.
A Comprehensive
Solution
One third of your employees living with diabetes also have a comorbidity, such as hypertension. That's why the OneTouch Reveal® Plus app includes features for blood pressure and weight management.
Two easy-to-access options combine the power of the OneTouch Reveal® Plus app with products from our #1 selling brand.
New! OneTouch® Direct
Along with the power of digital coaching, employees can now enjoy the convenience of free home delivery at no extra cost.
OneTouch® Preferred
Employees can support their self-care with OneTouch Reveal® Plus while continuing to pick up supplies at the pharmacy, just as they do today.
Class II Medical
Device
OneTouch Reveal® Plus is cleared by the FDA and designated as a Class II Medical Device.
Privacy &
Digital Security
OneTouch Reveal® Plus is SOC2 Certified, ISO13485 Certified and HIPAA Compliant.
Proven
Outcomes
Evidence-based clinical outcomes3,4. Economic outcome analysis conducted by IBM Watson Health.
What does investing in the OneTouch Reveal® Plus app look like?
Easy Payment
Options
We offer pricing options that fit your budget and performance indicator needs.
Flexible
Implementation
Our customized activation and engagement support provides seamless integration into your current program.
Innovation that
Supports Employees
We are constantly striving to delight and innovate with enhancements that evolve with your employees' needs.
Learn More
Activate the OneTouch Reveal® Plus app for your employees with diabetes today.
Let's Connect
The OneTouch Reveal® Plus app, powered by BlueStar®, is indicated for use by patients aged 18 and older who have Type 1 or Type 2 diabetes and their healthcare providers. The app is not intended to replace the care provided by a licensed healthcare professional. The app should not be used by patients with gestational diabetes or patients using an insulin pump. BlueStar® is a Welldoc, Inc. patented system. The OneTouch Reveal® Plus app is manufactured by Welldoc, Inc.
1www.diabetes.org/resources/statistics/cost-diabetes, December 2019
2Market Scan Analysis from Truven Analytics, an IBM Watson Health Company, for data collected between January 01, 2014 and December 31, 2015.
3Quinn CC, et al. Diabetes Care. 2011:34:1934-1942.
4Quinn CC, et al. Diabetes Technol Ther. 2008;10:160-168
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by LifeScan Scotland Ltd. and its affiliates is under license. Other trademarks and trade names are those of their respective owners.
US-OTRP-2000004